Fluorescent phenoxy benzoxazole complexes of zirconium and hafnium: synthesis, structure and photo-physical behaviour†
Abstract
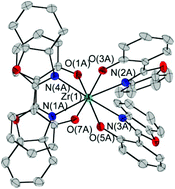
A series of ten heteroleptic and homoleptic mononuclear Zr(IV) and Hf(IV) complexes bearing differently substituted phenoxy-benzoxazole ligands was synthesised. The complexes were characterised by 1H and 13C{1H} NMR spectroscopy as well as by elemental analyses and X-ray diffraction experiments. The molecular structures show octahedral or tetragonal-antiprismatic coordination motifs at the metal atom. The crystal packing patterns depend on the substitution of the ligands. The intermolecular interactions in the solid state are governed by weak π–π interactions, and are also dependent on the ligand structure. Photo-physical investigations reveal that all ten complexes show a ligand-centered fluorescence with emission maxima in the blue region of the electromagnetic spectrum.


 Please wait while we load your content...
Please wait while we load your content...