Interception of intermediates in phosphine oxidation by mesityl nitrile-N-oxide using frustrated Lewis pairs†
Abstract
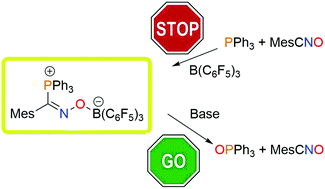
Phosphine oxidation by MesCNO is rapid; however, an FLP strategy intercepts the 1,3 addition products including [MesC(R3P)NOB(C6F5)3] (R = Ph 1, p-tol 2), [MesC(Mes2PH)NOB(C6F5)3] 3 (MesC(NOB(C6F5)3)Ph2P)2(CH2)n (n = 2: 4, 3: 5) and [MesC(Ph3P)NOB(C6F4H)3] 6. These species are shown to react with tBuOK or [Bu4N]F permitting the oxidation to proceed via a process involving borane dissociation. Similarly, the equilibrium established by 1 with B(C6F4H)3 and 6 with B(C6F5)3 provides experimental support for the “Cummins mechanism” for these phosphine oxidations.


 Please wait while we load your content...
Please wait while we load your content...