2D and 3D mixed MII/CuII metal–organic frameworks (M = Ca and Sr) with N,N′-2,6-pyridinebis(oxamate) and oxalate: preparation and magneto-structural study†
Abstract
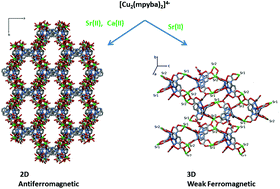
Three heterobimetallic complexes of formula [Ca2Cu3(mpyba)2(2-apyma)(H2O)7]·8.3H2O (1), [Sr2Cu3(mpyba)2(2-apyma)(H2O)8]·11.6H2O (2) and [Sr4.5Cu4(mpyba)4(ox)(H2O)20]·8.5H2O (3) [H4mpyba = N,N′-2,6-pyridinebis(oxamic acid), 2-apyma = 2-(6-aminopyridinyl)oxamate and ox = oxalate] have been synthesized and structurally characterized. Complexes 1 and 2 are isostructural compounds, with tricopper(II) units having mpyba and its hydrolytic product (2-apyma) as ligands. They are interlinked through strontium(II) (1) and calcium(II) (2) ions to afford neutral two-dimensional networks. Two of the copper(II) ions are five-coordinate in distorted square pyramidal (Cu3) and trigonal bipyramidal (Cu1) surroundings, whereas the other (Cu2) is six-coordinate in an elongated octahedral environment. The main difference between their structures, apart from the number of water molecules, resides in the nature of the alkaline earth cation coordinated to the oxamate fragments, Sr2+ (1)/Ca2+ (2), which exhibit eight and seven coordination, respectively. The π–π interactions and an extensive network of hydrogen bonds in 1 and 2 lead to supramolecular 3D structures. The relatively small size of their cavities, in the micropore domain, hinders the inclusion of N2 but allows CO2 adsorption (0.45 and 0.52 mmol g−1 for 1 and 2, respectively). The structure of 3 is made up of [3,3] metallacyclophane-type motifs, having the formula [Cu2(mpyba)2(H2O)2]4−. These act as tetrakis(bidentate) ligands towards the strontium(II) ions (Sr1, Sr2 and Sr3), leading to a sheet-like polymer growing in the bc plane, which extends further along the crystallographic a axis by a bis(chelating) oxalate between the Sr1 atoms. The investigation of the magnetic properties of 1–3 in the temperature range 1.9–300 K shows the occurrence of an overall antiferromagnetic behaviour for 1 and 2 [J12 = J23 = −9.71(2) (1) and −10.81(5) cm−1 (2), with the Hamiltonian being defined as H = −J12S1·S2 − J23S2·S3 + gβH[S1 + S2 + S3], and a ferromagnetic coupling within the dicopper(II) metallacylophane unit of 3 [J = +1.86(1) cm−1 through the Hamiltonian H = −JS1·S2 + gβH(S1 + S2)]. Simple orbital symmetry considerations (1–3) and the spin polarization mechanism (3) account for the observed magnetic properties.


 Please wait while we load your content...
Please wait while we load your content...