Bimetallic cooperative effect on O–O bond formation: copper polypyridyl complexes as water oxidation catalyst†
Abstract
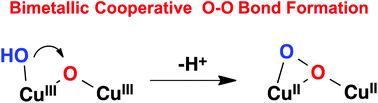
The performance of water oxidation catalysis by a Cu-based polypyridyl complex, [CuII(TPA)(OH2)]2+ (1H; TPA = tris-(pyridylmethyl)amine), has been investigated in neutral aqueous solution by electrochemical methods. Compared with our previously reported binuclear catalyst, [(BPMAN)(CuII)2(μ-OH)]3+ (2; BPMAN = 2,7-[bis(2-pyridylmethyl)aminomethyl]-1,8-naphthyridine), mononuclear catalyst 1 has a higher overpotential and lower catalytic activity toward water oxidation under the same conditions. Experimental results revealed that the O–O bond formation occurred via a water nucleophilic attack mechanism in which formal CuIV(O) is proposed as a key intermediate for the mononuclear catalyst 1H. In contrast, for the binuclear catalyst, O–O bond formation was facilitated by bimetallic cooperation between the two CuIII centers.


 Please wait while we load your content...
Please wait while we load your content...