Intrinsic alterations in the hydrogen desorption of Mg2NiH4 by solid dissolution of titanium
Abstract
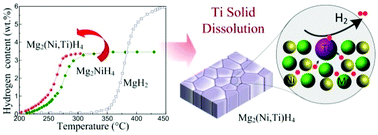
Achieving rapid hydrogen desorption at low temperatures is one of the main challenges for Mg-based alloys in hydrogen storage. Here, we report that the dehydrogenation process of Mg2NiH4 can be intrinsically improved with the solid dissolution of titanium into its lattices. The hydrogen desorption is suggested to be promoted by shifting the rate-limiting step from proven nucleation and/or diffusion to two-dimensional phase boundary migration based on kinetic modeling studies, for which few additions have been obtained, probably due to the understanding being confounded by other impurity phases. This alteration intrinsically results in enhanced desorption properties of Mg2NiH4 when Ti is dissolved, i.e., not only does it exhibit a decreased peak desorption temperature with a reduction in the activation energy, but it also positively changes the enthalpy value in comparison with that of the pure compound. These obtained results allow for a deep understanding of how the intrinsic desorption features are enhanced by solid dissolution, and also provide insight into improving hydrogen storage in Mg-based alloys.


 Please wait while we load your content...
Please wait while we load your content...