Serendipitous discoveries of new coordination modes of the 1,5-regioisomer of 1,2,3-triazoles enroute to the attempted synthesis of a carbon-anchored tri-mesoionic carbene†
Abstract
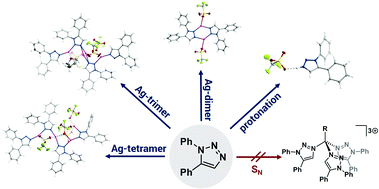
We report herein the synthesis and solid state structures of a protonated 1,5-diphenyl-1,2,3-triazole and a hydroxyl–alkyl-substituted 1,5-diphenyl-1,2,3-triazolium salt. Furthermore, we present a simple silver(I) triflate–triazole adduct, which forms di-, tri- and tetranuclear complexes depending on the crystallization conditions. The 1,5-regioisomer of the triazole acts as a bridging as well as a terminal ligand in these structures, coordinating via N2 as well as N3 nitrogen donors. These observations underline the versatility in coordination chemistry of this underused ligand class. All observations were made while attempting the synthesis of a carbon-anchored tripodal mesoionic carbene ligand. Apart from showcasing the coordination flexibility and the versatility of this underused regioisomer, we also present sound evidence for the organic products obtained during the attempted synthesis of the tri-mesoionic carbene. The latter results might be insightful in future synthesis of this elusive molecule.


 Please wait while we load your content...
Please wait while we load your content...