1,4-Dihydropyridyl complexes of magnesium: synthesis by pyridine insertion into the magnesium–silicon bond of triphenylsilyls and catalytic pyridine hydrofunctionalization†
Abstract
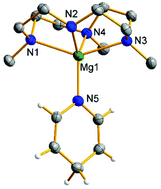
Magnesium bis(triphenylsilyl) [Mg(SiPh3)2(THF)2]·THF (1) reacted with a stoichiometric amount of pyridine to give the magnesium 4-(triphenylsilyl)dihydropyridyl complex [Mg(NC5H5-4-SiPh3)2(THF)3] (2). Using an excess of pyridine, a mixture of magnesium dihydropyridyl [Mg(NC5H6)2(py)4] (3) and 4-(triphenylsilyl)pyridine was formed. Complex 3 underwent exchange with pyridine-d5 at 25 °C to give [Mg(NC5D5H-4)2(py-d5)4] (3-HD). Analogous reactions with Me3TACD-supported magnesium triphenylsilyls [(Me3TACD)Mg(SiPh3)] (4) and [(Me3TACD·AlEt3)Mg(SiPh3)] (6) ((Me3TACD)H = Me3[12]aneN4: 1,4,7-trimethyl-1,4,7,10-tetraazacyclododecane) with pyridine gave [(Me3TACD)Mg(NC5H5-4-SiPh3)] (5), [(Me3TACD·AlEt3)Mg(NC5H5-4-SiPh3)] (7) and a mixture of [(Me3TACD)Mg(NC5H6)] (8) and 4-(triphenylsilyl)pyridine. Complex 8 is also formed by reacting 3 with (Me3TACD)H and underwent exchange with pyridine-d5 at higher temperatures. The activation energy for the exchange is about 25 kJ mol−1 higher than that for the exchange reaction of 3 to 3-HD. Complexes 2, 3, 3-HD, 5, 7 and 8 were characterized by NMR spectroscopy and 3, 5 and 8 by single crystal structure analysis. Complex 3 was found to be slightly active in the hydrosilylation of pyridine using phenylsilane, whereas complex 8 showed no activity. Both complexes 3 and 8 were active in the hydroboration of pyridine with pinacolborane.
- This article is part of the themed collection: Organometallic and coordination chemistry of the s-block metals


 Please wait while we load your content...
Please wait while we load your content...