Weakly interacting solvation spheres surrounding a calixarene-protected tetrairidium carbonyl cluster: contrasting effects on reactivity of alkane solvent and silica support†
Abstract
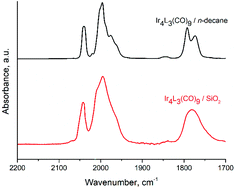
The tetrairidium carbonyl cluster Ir4L3(CO)9 (L = tert-butyl-calix[4]arene(OPr)3(OCH2PPh2) (Ph = phenyl; Pr = propyl)) on a partially dehydroxylated silica support undergoes hydrogen activation at a rate and with a mechanism different from those pertaining to the cluster in alkane solution. These results are unobvious in view of the sterically bulky ligands protecting the cluster and the nearly identical CO band frequencies in the infrared spectra characterizing the supported and dissolved Ir4L3(CO)9, both before reaction and during reaction involving decarbonylation in the presence of either helium or H2 (and H2 reacted with the clusters to form hydrides with the same Ir–H band frequencies for clusters in alkane solvent and supported on silica). The initial rates of CO loss from the supported clusters in the presence of helium were the same as those in the presence of H2. The comparison demonstrates that the rate-determining step for hydride formation on the silica-supported cluster is CO dissociation. In contrast, the comparable dissociation of CO from the cluster in n-decane solution requires a higher temperature, 343 K, and is at least an order of magnitude slower than when the clusters were supported on silica. CO dissociation is not the rate-determining step for hydrogen activation on the cluster in n-decane, as the rate is influenced by reactant H2 as well.


 Please wait while we load your content...
Please wait while we load your content...
