Air and moisture stable covalently-bonded tin(ii) coordination polymers†
Abstract
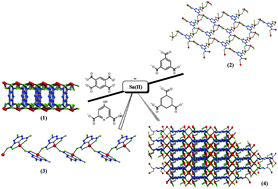
Four covalently-bonded tin(II) coordination polymers, (1)–(4), were hydrothermally prepared in aqueous alkaline media by the reactions of SnSO4 with 1,2,4,5-benzenetetracarboxylic acid (1), 1,3,5-benzenetricarboxylic acid (2), 4-hydroxypyridine-2,6-dicarboxylic acid (3), and 1,3,5-cyclohexanetricarboxylic acid (4). All products were structurally authenticated by single-crystal X-ray diffraction, and the number of different tin centres and their oxidation states were confirmed by 119Sn Mössbauer spectroscopy. In addition, the comparison between experimental and simulated X-ray powder diffraction patterns confirmed the authenticity of the samples. Our crystallographic results for (1)–(4) show that the Sn(II) centres are tetracoordinated and exhibit distorted disphenoidal geometries, corroborating the presence of one stereochemically active lone electron pair at each metal site. Products (1) and (2) display bi-dimensional polymeric structures, (3) exhibits a one-dimensional architecture, whereas (4) shows a remarkable three-dimensional coordination network. Hirshfeld surface and supramolecular analyses for the repeating units of (1)–(4) were also performed in order to identify structurally important non-covalent interactions.


 Please wait while we load your content...
Please wait while we load your content...