Promoting effect of nickel hydroxide on the electrocatalytic performance of Pt in alkaline solution†
Abstract
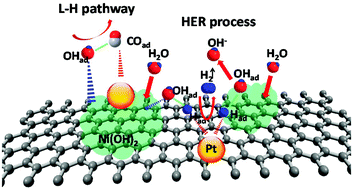
Despite intense research in the past decades, the lack of high-performance catalysts for fuel cell reactions remains a challenge in realizing fuel cell applications. Herein, we report a novel hybrid nanomaterial of platinum–nickel hydroxide–nanotubes (Pt/Ni(OH)2/CNTs) for improving electrocatalytic performance in alkaline environments. Ni(OH)2 was directly grown on functionalized nanotubes and then, Pt nanoparticles were in situ immobilized by the microwave synthesis method. Due to electronic and synergistic effects, 10 : 2–Pt/Ni(OH)2/N-CNT catalyst exhibited 2.77 times specific activity and 6.27 times mass activity toward methanol oxidation reaction (MOR), which were higher than those of commercial Pt/C in alkaline solution. The CO-stripping experiments and hydrogen evolution reaction (HER) further demonstrated that Ni(OH)2 could promote oxidation removal of carbonaceous poison for MOR via accelerating water dissociation: (i) Ni(OH)2 acted on an H2O molecule, leading to the formation of OHad; (ii) OHad oxidized the intermediate COad to CO2. Furthermore, the 10 : 2–Pt/Ni(OH)2/N-CNT catalyst also exhibited 2.07 times specific activity and 1.67 times mass activity toward oxygen reduction reaction (ORR), which were higher than those of commercial Pt/C in alkaline solution and Pt/N-CNT catalysts. Thus, the preparation of this hybrid nanomaterial provides a new direction for catalyst performance optimization towards next-generation fuel cells in alkaline environments.


 Please wait while we load your content...
Please wait while we load your content...