Complete charge separation provoked by full cation encapsulation in the radical mono- and di-anions of 5,6:11,12-di-o-phenylene-tetracene†
Abstract
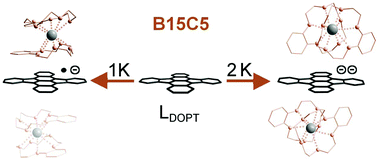
Herein, we report the synthesis and molecular structure of the mono- and dianionic aromatic molecules [(B15C5-κ5O)2K+](LDOPT˙−) (1) and [(B15C5-κ5O)2K+]2(LDOPT2−)THFsolv (2) derived from the parent aromatic polyhydrocarbon 5,6:11,12-di-o-phenylenetetracene (DOPT, LDOPT) by a controlled stepwise one and two electron chemical reduction. The effect of single and double electron charge transfer to a polycondensed aromatic hydrocarbon (PAH) without any disturbing influence of an associated metal cation has been demonstrated. This was achieved by fully sandwiching the cationic K+ counterions between two benzo-15-crown-5-ether (B15C5) ligands resulting in a fully encapsulating (κ10O) geometry which ensures a complete separation of the K+ counterions and the bare anionic PAH species [LDOPT˙−] and [LDOPT2−]. The structural changes accompanied by the stepwise reduction from LDOPT to [LDOPT˙−] to [LDOPT2−] are discussed and compared to earlier predictions based on density functional theory (DFT) as well as the results of previous studies of alkaline metal cationic PAH anion interactions of DOPT in which only a partial metal cation encapsulation has been achieved so far.


 Please wait while we load your content...
Please wait while we load your content...