Reactivity of a dearomatised pincer CoIIBr complex with PNCNHC donors: alkylation and Si–H bond activation via metal–ligand cooperation†‡
Abstract
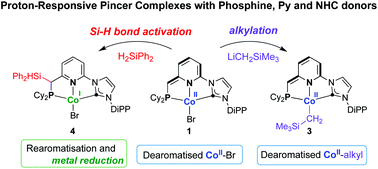
The double aminolysis reaction of [Co{N(SiMe3)2}2] by the salt 1-(6-((dicyclohexylphosphaneyl)methyl)pyridin-2-yl)-3-(2,6-diisopropylphenyl)-1H-imidazol-3-ium bromide, which contains one phosphane, one pyridine and one imidazolium groups, of formula [o-Cy2PCH2(C5H3N)(o-C3H3N2DiPP)]Br and abbreviated as (CyPNpyrCim)Br, was previously shown to afford the Co(II) complex [Co(CyP*NaCNHC)Br] (1) containing a dearomatised picolyl moiety in the tridentate, anionic donor ligand CyP*NaCNHC (Na = anionic amido, P* = vinylic P donor). We now report that formation of 1 is preceded by an intermediate tentatively assignable to the 5-coordinate Co(II) complex [Co(CyPNpyrCNHC){N(SiMe3)2}Br] (2). The reaction of 1 with LiCH2SiMe3 afforded the dark purple, paramagnetic [Co(CyP*NaCNHC)CH2SiMe3] (3) with a low spin d7 CoII; the electronic configurations of 1 and 3 were corroborated by EPR spectroscopy. Addition of excess (≥4 equiv.) H2SiPh2 to a solution of 1 gave the diamagnetic [Co(CyP(SiHPh2)NCNHC)Br] (4) following Si–H activation and silylation of the ligand backbone at the α-CHP. Reduction of CoII to CoI by silanes is uncommon.


 Please wait while we load your content...
Please wait while we load your content...