Intramolecular metal–ligand electron transfer triggered by co-ligand substitution†
Abstract
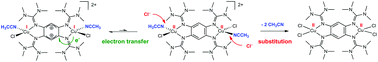
The possibility of directed stimulation of intramolecular electron transfer between a metal and a redox-active ligand in a molecular coordination compound is the key to its application in molecular catalysis and other research themes. Although the stimulation by a substitution reaction of the co-ligands is often postulated as key step in catalytic cycles using redox-active ligands as electron reservoirs, there are only a few explicit examples for such reactions. Herein we report the synthesis of the first dicationic and dinuclear CuI complexes featuring the oxidized form of a redox-active tetrakisguanidine ligand (1,2,4,5-tetrakis(tetramethylguanidino)benzene 1) as a bridging ligand and two neutral co-ligands L (acetonitrile or pyridine), [1{Cu(Cl)L}2]2+. An intramolecular electron transfer between the copper atom and the tetrakisguanidine ligand 1, leading to a dinuclear CuII complex with the reduced form of the tetrakisguanidine ligand 1, is triggered by substitution of the neutral co-ligands L.


 Please wait while we load your content...
Please wait while we load your content...