Chemo-chromism in an orthogonal dabco-based Co(ii) network assembled by methanol-coordination and hydrogen bond formation†
Abstract
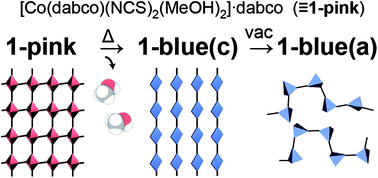
Newly prepared single crystals of [Co(II)(dabco)(NCS)2(MeOH)2]·dabco (1-pink; dabco: 1,4-diazabicyclo[2.2.2]octane) showed chromotropic behaviour in the solid state, changing from pink to blue upon heating or grinding. The complex 1-pink exhibited a two-dimensional orthogonal network structure with the coordination chain of –dabco–Co– bridged by hydrogen bonds between coordinative methanol and a second dabco molecule, where the methanol molecule was trapped by coordinative and hydrogen bonds. Chromism was demonstrated to stem from the quantitative desorption of methanol from 1-pink to produce [Co(II)(dabco)(NCS)2]·dabco (1-blue(c)) by thermogravimetric (TG) and temperature controlled gas chromatography-mass spectrometry (GC-MS) analyses, and powder X-ray diffraction (XRD) analysis suggests that the transformation between the crystalline phases of 1-pink and 1-blue(c) occurred with similar lattice parameters. Furthermore, the desolvated species showed chemo-chromic behaviour due to the selective size- and polarity-dependent adsorption of solvent molecules.


 Please wait while we load your content...
Please wait while we load your content...