Solvent-dependent variations of both structure and catalytic performance in three manganese coordination polymers†
Abstract
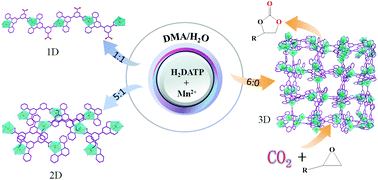
Three new manganese 4′-(3,5-dicarboxyphenyl)-2,2′:6′,2′′′-terpyridine (H2DATP) metal–organic framework materials have been generated through regulating the ratios of a binary solvent mixture (DMA/H2O) under solvothermal conditions. Compound 1 {[Mn2(DATP)(HDATP)(H2O)4](OH)·10H2O}n displaying a one-dimensional (1D) chainlike structure was crystallized from the DMA/H2O mixture with a molar ratio of 1 : 1, while the two-dimensional (2D) layer species, {[Mn(DATP)(H2O)]·2H2O}n (2) was produced by increasing the ratio of DMA/H2O to 5 : 1. Interestingly, the crystallization in pure DMA yields a three-dimensional (3D) interpenetrating network {[Mn(DATP)]·4H2O}n (3), featuring higher solvent stability and pH stability than compounds 1 and 2. It is proved that solvent not only influences the structural transformation process of crystals but also has a significant effect on their properties. These three compounds present different catalytic performances in the CO2 cycloaddition to epoxides with various substituent groups into corresponding cyclic carbonates, and only 3 can serve as an efficient and recyclable catalyst at mild temperature.


 Please wait while we load your content...
Please wait while we load your content...