Phosphine phosphonic amide nickel catalyzed ethylene polymerization and copolymerization with polar monomers†
Abstract
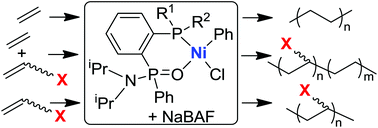
The phosphine phosphonic amide ligand platform is highly versatile, with three positions that can be independently tuned. In this contribution, we wish to study the nickel complexes based on this ligand system. Interestingly, the nickel dibromide and nickel allyl complexes are not active in ethylene polymerization. In contrast, the nickel phenyl chloride complexes are highly active in ethylene polymerization in the presence of a sodium tetrakis(3,5-bis(trifluoromethyl)phenyl)borate cocatalyst. In addition, these nickel complexes can initiate ethylene copolymerizations with polar functionalized comonomers including methyl 10-undecenoate, 6-chloro-1-hexene and 5-acetoxy-1-pentene. More interestingly, these nickel complexes can oligomerize 1-hexene and 6-chloro-1-hexene.


 Please wait while we load your content...
Please wait while we load your content...