Supported ammonia borane decomposition through enhanced homopolar B–B coupling†
Abstract
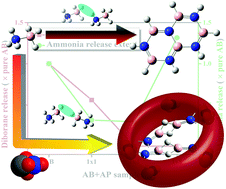
The thermolytic decomposition of ammonia borane (AB) is known to proceed through the polymeric coupling reaction between –BH3 and –NH3 sites of multiple ammonia borane molecules, which results in the release of hydrogen and other by-products, e.g., ammonia, diborane and borazine. The formation of these by-products concomitantly pollutes the hydrogen stream, and therefore, it is necessary to remove these gases from the product stream. In the current work, a cost effective and easy to synthesize support material, aluminium phosphate (AP), is introduced in AB thermolytic decomposition. An in situ MS study reveals that the AB and AP (w/w) loading ratio of (1 × 4) is the most promising as it is able to minimise the dehydrogenation peak temperature by 18.89 °C compared to that of pure AB. Additionally, in the presence of support material, the by-product formation of ammonia is reduced by 70.3%, with a complete suppression in borazine and diborane release. The mechanism behind the by-product suppression of supported AB has been studied through 11B MAS NMR analysis which suggests that the release of hydrogen occurs through an intermolecular homopolar B–B bonding. The thermogravimetric and kinetic study also reveals that in the case of supported AB decomposition, hydrogen release through B–B interaction is much more efficient than a B–N interaction, thus limiting the possibility of autocatalysis during the supported decomposition reaction.


 Please wait while we load your content...
Please wait while we load your content...