Efficient electrocatalytic hydrogen gas evolution by a cobalt–porphyrin-based crystalline polymer†
Abstract
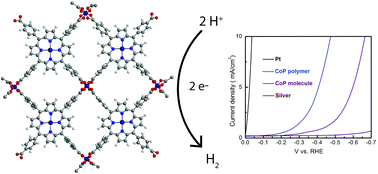
Herein, we report a crystalline CoTcPP-based [TcPP = the anion of meso-tetra(4-carboxyphenyl)porphyrin] polymeric system, 1, as a hydrogen evolution reaction (HER) electrocatalyst in acidic aqueous media. The material was characterized by powder X-ray diffraction (p-XRD), Fourier transform infrared (FT-IR) spectroscopy, and energy dispersive X-ray (EDX) analysis and its morphology was probed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Polymer 1 shows a surface area of 441.74 m3 g−1, while the discrete CoTcPP molecule (2) shows a surface area of 3.44 m3 g−1. The HER catalytic performance was evaluated by means of linear sweep voltammetry in the presence of 0.5 M H2SO4 aqueous solution. To achieve 10 mA cm−2 cathodic current density, 1 and 2 respectively require an overpotential of 0.475 V and 0.666 V, providing strong evidence that the extended network of cobalt-based porphyrin leads to enhanced HER efficiency. The polymer also shows great tolerance for HER electrolysis in the presence of an acid remaining active over 10 hours.


 Please wait while we load your content...
Please wait while we load your content...
