Thermodynamic description of Tc(iv) solubility and carbonate complexation in alkaline NaHCO3–Na2CO3–NaCl systems†
Abstract
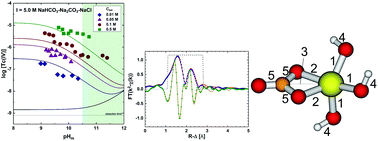
The solubility of 99Tc(IV) was investigated in dilute to concentrated carbonate solutions (0.01 M ≤ Ctot ≤ 1.0 M, with Ctot = [HCO3−] + [CO32−]) under systematic variation of ionic strength (I = 0.3–5.0 M NaHCO3–Na2CO3–NaCl–NaOH) and pHm (−log[H+] = 8.5–14.5). Strongly reducing conditions (pe + pHm ≈ 2) were set with Sn(II). Carbonate enhances the solubility of Tc(IV) in alkaline conditions by up to 3.5 log10-units compared to carbonate-free systems. Solvent extraction and XANES confirmed that Tc was kept as +IV during the timeframe of the experiments (≤ 650 days). Solid phase characterization performed by XAFS, XRD, SEM-EDS, chemical analysis and TG-DTA confirmed that TcO2·0.6H2O(am) controls the solubility of Tc(IV) under the conditions investigated. Slope analysis of the solubility data in combination with solid/aqueous phase characterization and DFT calculations indicate the predominance of the species Tc(OH)3CO3− at pHm ≤ 11 and Ctot ≥ 0.01 M, for which thermodynamic and activity models are derived. Solubility data obtained above pHm ≈ 11 indicates the formation of previously unreported Tc(IV)–carbonate species, possibly Tc(OH)4CO32−, although the likely formation of additional complexes prevents deriving a thermodynamic model valid for this pHm-region. This work provides the most comprehensive thermodynamic dataset available for the system Tc4+–Na+–Cl−–OH−–HCO3−–CO32−–H2O(l) valid under a range of conditions relevant for nuclear waste disposal.


 Please wait while we load your content...
Please wait while we load your content...