Regulating structural dimensionality and emission colors by organic conjugation between SmIII at a fixed distance†
Abstract
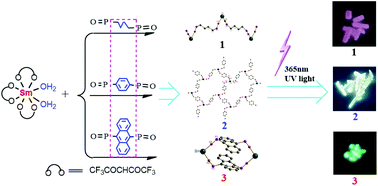
The conjugation of bridging bis(diphenylphosphine oxide) alkane or arene ligands was found to control the structural dimensionality and the emission color of complexes from reactions with SmIII(hfac)3(H2O)2 (hfac− = hexafluoroacetylacetonato) while retaining the Sm⋯Sm distances. Bis(diphenylphosphine oxide)-1,4-butane (L1) affords a one-dimensional (1D) ribbon {Sm(hfac)3(L1)}∞ (1) that emits red color, while bis(diphenyl-phosphinoyl)-1,4-benzene (L2) results in a two-dimensional (2D) network {Sm(hfac)2(CF3COO)(L2)3}∞ (2) and near-white emission, but bis(diphenyl-phosphinoyl)-9,10-anthracene (L3) forms a zero-dimensional (0D) cyclic structure {Sm(hfac)3(L3)}2 (3) with strong π⋯π interactions that emit green color. Noticeably, the conjugation change is accompanied by a configurational change of coordination from trans for 1 and 2 to cis for 3. The color change is associated with the superposition of ligand and Sm based electronic band energies and their intensities. Such white light emission by a single compound having contributions from different building components is quite rare.


 Please wait while we load your content...
Please wait while we load your content...