Mitochondria-localising DNA-binding biscyclometalated phenyltriazole iridium(iii) dipyridophenazene complexes: syntheses and cellular imaging properties†
Abstract
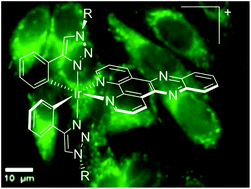
Two new biscyclometalated complexes [Ir(ptzR)2(dppz)]+ (dppz = dipyridophenazene; ptzRH = 4-phenyl-1-benzyl-1,2,3-triazole (1+) and 4-phenyl-1-propyl-1,2,3-triazole (2+)) have been prepared. The hexafluorophosphate salts of these complexes have been fully characterized and, in one case, the X-ray structure of a nitrate salt was obtained. The DNA binding properties of the chloride salts of the complexes were investigated, as well as their cellular uptake by A2780 and MCF7 cell lines. Both complexes display an increase in the intensity of phosphorescence upon titration with duplex DNA, indicating the intercalation of the dppz ligand and, given that they are monocations, the complexes exhibit appreciable DNA binding affinity. Optical microscopy studies reveal that both complexes are taken up by live cancer cell lines displaying cytosol based luminescence. Colocalization studies with commercial probes show high Pearson coefficients with mitotracker dyes confirming that the new complexes specifically localize on mitochondria.


 Please wait while we load your content...
Please wait while we load your content...