Combining covalent bonding and electrostatic attraction to achieve highly viable species with ultrashort beryllium–beryllium distances: a computational design†
Abstract
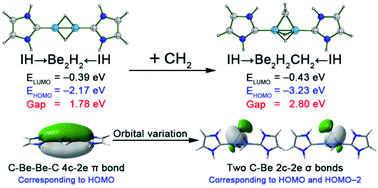
Though ultrashort metal–metal distances (USMMD, dM–M < 1.900 Å) were primarily realized between transition metals, USMMDs between main group metal atoms such as beryllium atoms have also been designed previously using two strategies: (1) formation of multiple bonding orbitals or (2) having favourable electrostatic attraction. We recently turned our attention to the reported species IH → Be2H2 ← IH (where IH denotes imidazol-2-ylidene) because the orbital energy level of its π-type HOMO is noted to be very high, which may result in intrinsic instability. In the present study, we combined the abovementioned strategies to solve the high orbital energy level problem without losing the ultrashort Be–Be distances. It was found that breaking of such π-type HOMO by addition of a –CH2– group onto the bridging position of two beryllium atoms led to the formation of IH → Be2H2CH2 ← IH species, which not only possesses an ultrashort Be–Be distance in the –Be2H2CH2– moiety, but also has a relatively low HOMO energy level. Replacing the IH ligands with NH3 and PH3 resulted in the formation of NH3 → Be2H2CH2 ← NH3 and PH3 → Be2H2CH2 ← PH3 species with similar features. The electronic structure analyses suggest that the ultrashort Be–Be distances in these species are achieved by the combined effects of the formation of two Be–H–Be 3c-2e bonds and having favourable Coulombic attractions between the carbon atom of the –CH2– group and two beryllium atoms. Remarkably, when the IH, NH3, and PH3 ligands were replaced by large ligands with bulky groups, such as 1,3-bis(2,6-diisopropyl phenyl)imidazol-2-ylidene (IDip), triphenylamine (NPh3), and triphenylphoshpine (PPh3), respectively, the resultant species IDip → Be2H2CH2 ← IDip, NPh3 → Be2H2CH2 ← NPh3, and PPh3 → Be2H2CH2 ← PPh3 exhibit good steric protection around the –Be2H2CH2– core. These species are thus examples for the experimental realization of species with ultrashort metal–metal distances between main group metals.


 Please wait while we load your content...
Please wait while we load your content...