Making organoruthenium complexes of 8-hydroxyquinolines more hydrophilic: impact of a novel l-phenylalanine-derived arene ligand on the biological activity†
Abstract
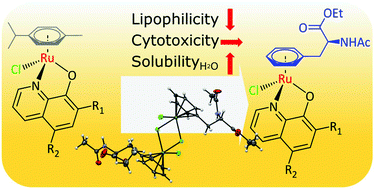
Ru(arene) compounds have many desirable features making them promising candidates for further development in anticancer drug research. While a lot of emphasis has been placed on the modification of the ancillary ligands, there are not many examples of arene ligands bearing functional groups. Herein, we report the preparation of [Ru(arene)(8-oxyquinolinato)Cl] complexes with the arene being a protected form of the amino acid L-phenylalanine and 8-oxyquinolinato ligand substituted with halogens. With this approach we aimed to alter the pharmacological properties of the complexes and address issues with the aqueous solubility of the analogous p-cymene complexes. The complexes were shown to be stable in DMSO and water and reacted readily with L-histidine and 9-ethylguanine as protein and DNA models, respectively. Assaying the antiproliferative activity in cancer cells gave IC50 values in the low μM range. While the lipophilicity of the p-cymene analogues correlated well with their in vitro cytotoxicity, the potency of the complexes with the L-phenylalanine-derived arene was independent of lipophilicity.


 Please wait while we load your content...
Please wait while we load your content...