Tuning the nucleophilicity of electron-rich diborane(4) compounds with bridging guanidinate substituents by substitution†
Abstract
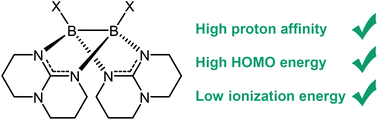
Diborane(4) compounds are versatile reagents in synthetic chemistry. Generally, diboranes(4) with sp2-hybridized boron atoms react as electrophiles. By contrast, the chemistry of nucleophilic diborane(4) compounds with two sp3-hybridized boron atoms is very much underdeveloped. In this work, we systematically vary the substituents of electron-rich diborane(4) compounds with bridging guanidinate substituents. In this way, five new diboranes are synthesized and fully characterized. Using quantum chemical computations, we show that the electronic properties and reactivity of these compounds can be rationally varied by the choice of substituents. The HOMO energies, adiabatic ionization energies and proton affinities are considered as parameters to compare the chemical properties of these unusual compounds.


 Please wait while we load your content...
Please wait while we load your content...