A new heptadentate picolinate-based ligand and its corresponding Gd(iii) complex: the effect of pendant picolinate versus acetate on complex properties†
Abstract
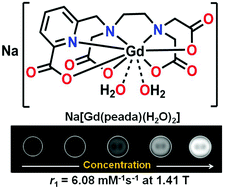
To attain high relaxivity as well as stability, a new water-soluble, water-coordinated Gd(III) complex, which was synthesised by reacting equimolar amounts of picolinate-based ligand H4peada and GdCl3·xH2O at pH ∼ 6.5, was examined. The number of inner sphere water molecules (q) in the complex was found to be 1.7 ± 0.1 from luminescence lifetime measurements of its Tb(III) congener, complex 2. At 1.41 T, 25 °C, and pH = 7.4, the longitudinal relaxivity (r1) value of the complex was found to be 6.08 mM−1 s−1, which remained almost constant in the pH range 4–10. The r1 relaxivity value has not been affected in the presence of a 100 fold excess of bicarbonate and phosphate anions, whereas in the case of fluoride ions, the value dropped to 4.6 mM−1 s−1 due to a binding interaction of fluoride ions by replacing inner sphere water molecules of the complex. From the potentiometric titration method, the stability constant of the complex was found to be log KGdL = 17.0 ± 0.08 (in 0.15 M KCl and 25 °C). At pH = 7.4, the pGd value of ligand H4peada was found to be 14.01 which was comparable to the commercially available MRI contrast agent Omniscan®. Phantom MR images of the complex under a clinical MR scanner at 1.5 T also demonstrated the usefulness of complex 1 as a potential MRI contrast agent.


 Please wait while we load your content...
Please wait while we load your content...