Synthesis, spectroscopic characterisation and transmetalation of lithium and potassium diaminophosphanide-boranes†‡
Abstract
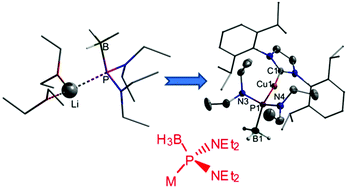
A secondary diaminophosphane-borane (Et2N)2PH(BH3) was prepared from a chlorophosphane precursor and LiBH4 and metalated by reaction with anion bases (n-BuLi, KN(SiMe3)2) to yield the corresponding metal diaminophosphanide-boranes [(Et2N)2P(BH3)]M (M = Li, K). Multinuclear NMR studies permitted the first spectroscopic characterisation of the metalation products and revealed the presence of monomeric (for M = Li) contact ion pairs in solution. NMR spectroscopic evidence that the ions in each pair interact via Li⋯P- rather than Li⋯H3B-interactions as had been inferred for a Ph-substituted analogue was confirmed by DFT studies, which revealed also that the borane coordination plays a decisive role in boosting the PH-acidity of the original secondary diaminophosphane precursor. Transmetalation of the potassium and lithium diaminophosphanide-boranes with Cu(I) and Zn(II) chlorides afforded the first functional transition metal complexes of a P-heteroatom-functionalised phosphanide-borane ligand. Both products were fully characterised. Thermolysis of the Cu-complex induced a reaction which involved transfer of an NHC ligand from the metal to the phosphorus atom and yielded a phosphaalkene NHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PH (NHC = N-heterocyclic carbene) as the major phosphorus-containing product.
PH (NHC = N-heterocyclic carbene) as the major phosphorus-containing product.
- This article is part of the themed collection: Philip Power at 65: an icon of organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...