Facile one-pot green synthesis of Au–Ag alloy nanoparticles using sucrose and their composition-dependent photocatalytic activity for the reduction of 4-nitrophenol†
Abstract
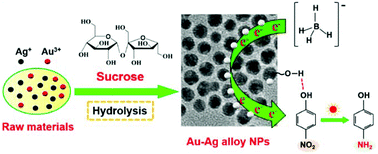
Au–Ag alloy nanoparticles (NPs) less than 10 nm in size were synthesized using sucrose as a reductant and surfactant. Au–Ag alloy NPs with a homogeneous composition were continuously obtained by changing the synthesis time from 2 to 40 min in one pot. Based on the UV-Vis, ICP, TEM, HR-TEM, EDX and SAED analyses, the synthesis mechanism of Au–Ag alloy NPs was deduced. Under hydrolysis conditions, sucrose showed a stronger reducibility compared with glucose, fructose and their mixture. And the as-prepared Au–Ag alloy NPs exhibited a superior photocatalytic activity and stability for the reduction of 4-nitrophenol due to the abundant hydroxyl groups of sucrose and the synergistic effect between Au and Ag elements. The rate constant of 4-nitrophenol reduction could be linearly controlled by the composition of Au–Ag alloy NPs or their synthesis time. It was indicated that the photocatalytic activity of Au–Ag alloy NPs could be predetermined as early as their synthesis process. The above methods of controlling the rate constant provide promising routes for other photocatalytic reactions using bimetallic NPs as photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...