Effects of nucleophilic ligands on the chain initiation efficiency of polar monomer polymerizations catalyzed by 2-methoxyethylaminobis(phenolate)yttrium complexes: a DFT study†
Abstract
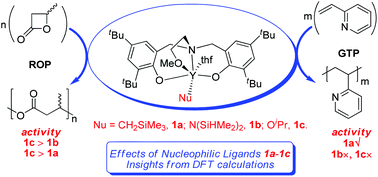
2-Methoxyethylaminobis(phenolate)yttrium complexes Y(ONOOtBu)(Nu)(THF) have unique catalytic activity and selectivity toward the ring-opening polymerization (ROP) of β-butyrolactone (BBL). Some of them also showed activity toward the group-transfer polymerization (GTP) of Michael-type monomers. These complexes with various nucleophilic ligands (Nu = CH2SiMe3, 1a; N(SiHMe2)2, 1b; OiPr, 1c) showed discrepant activity for ROP and GTP. The chain initiation of the ROP of BBL and the GTP of 2-vinylpyridine (2VP) catalyzed by these complexes is investigated by DFT calculations. The results indicate that, in the case of the ROP of BBL, less steric hindrance of the nucleophilic anionic ligand OiPr makes complex 1c have a higher chain initiation efficiency and thus a higher polymerization activity than 1a and 1b. In the case of the GTP of 2VP, the activity of 1a benefits from a good orbital match between the moieties of Y–CH2SiMe3 and vinyl of 2VP. The origin of the experimentally observed inertness of 1b has also been elucidated. These results could add to the better understanding of the experimentally observed activity discrepancy and shed light on the development of such a catalysis system.


 Please wait while we load your content...
Please wait while we load your content...