Chasing the agostic interaction in ligand assisted cyclometallation reactions of palladium(ii) †
Abstract
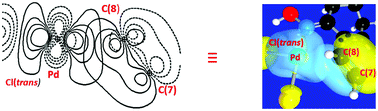
A 500 MHz NMR study of the reaction between 1-tetralone oxime and PdCl42− in CD3OD shows resonances attributable to a potential agostic intermediate prior to the formation of the insoluble cyclopalladated product which itself was characterised by X-ray crystallography. Calculated structural, spectroscopic, QTAIM, NBO and NCI analysis results obtained from density functional theory (DFT) calculations give a full description of the putative agostic intermediate [PdCl2(1-tetralone oxime)] (1) which is shown to include a previously unrecognised π-electron density donation from the aromatic ring to the metal in close proximity to the agostic carbon atom. Changing the (N)–OH donor to (N)–OMe does not effect the magnitude of these interactions. (N)–OH and (N)–OMe acetophenone imines in which the aromatic ring has the potential to rotate show similar agostic and π-electron donation to the alicyclic ring counterparts. 1-Tetralone which coordinates to the metal by a Pd–O bond that is much weaker than the Pd–N complexes has a slightly stronger agostic component and slightly weaker π-electron donation than the oxime counterpart.


 Please wait while we load your content...
Please wait while we load your content...