Protonation and electrochemical reduction of rhodium– and iridium–dinitrogen complexes in organic solution†
Abstract
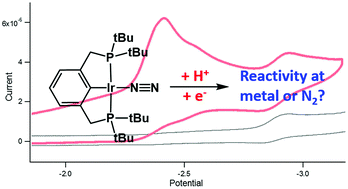
Protonation and reduction of pincer-ligated Rh– and Ir–N2 complexes have been studied by NMR spectroscopy and cyclic voltammetry to assess the capability of these complexes to activate or reduce N2. Protonation, which is a prerequisite to electrochemical reduction, results in a cationic metal-hydride that loses N2 under an atmosphere of Ar. Reduction of the metal-hydride results in fast disproportionation of an unobserved transient Ir2+ species. These studies suggest that the regioselectivity of initial protonation is a strong determinant for the ability of a system to facilitate the reduction of N2.


 Please wait while we load your content...
Please wait while we load your content...