Synthesis and reactivity of thiolate-bridged multi-iron complexes supported by cyclic (alkyl)(amino)carbene†
Abstract
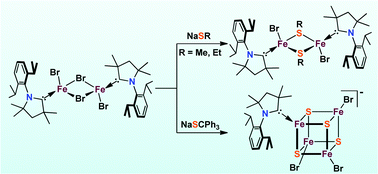
The combined utilization of Me2-cAAC (Me2-cAAC = :C(CH2)(CMe2)2N-2,6-iPr2C6H3) and thiolates as supporting ligands enables the access of unprecedented carbene coordinated thiolate-bridged diiron(II) complexes [(Me2-cAAC)Fe(μ-SR)(Br)]2 (R = Me, 3; R = Et, 4). The coordination environment of each tetrahedral iron(II) center in complexes 3 and 4 is composed of one terminal bromide atom, one carbene carbon atom and two thiolate sulfur atoms, which is similar to the carbide-containing sulfur-rich environment of iron centers in the belt region of the FeMo-cofactor. Interestingly, when NaSCPh3 was chosen as the thiolate ligand, C–S bond homolysis occurred to form a rare [3 : 1] site-differentiated cubane-type cluster [(Me2-cAAC)Fe4S4(Br)3][Me2-cAACH] (5). Furthermore, complexes 3 and 4 exhibit good exchange reactivity toward the azide anion to give novel thiolate-bridged diiron complexes with two azido ligands in a trans arrangement.


 Please wait while we load your content...
Please wait while we load your content...