Synthesis and oxidation of phosphine cations†
Abstract
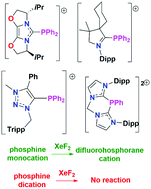
Cationic phosphines of the form [(L)PPh2]+ are prepared by reaction of Ph2PCl with carbenes (L) including a chiral bis(oxazoline)-based carbene, a cyclic(alkyl)(amino) carbene (cAAC), and a 1,2,3-triazolium-derived carbene, affording the products, [(IBox-iPr2)PPh2][OSO2CF3] 1, [(cAAC)PPh2][OSO2CF3] 2 and [((TripCH2N2(NMe)C2Ph)PPh2)2(AgCl)2][Cl]23. Using PhPCl2, the related dication [CH2(NC3H2NDipp)2PPh]2+4 was also prepared. Crystallographically-determined metric parameters and computational data indicate that these species are best described as cationic phosphines rather than phosphenium cations. The oxidation of these cations with XeF2 afforded [(IBox-iPr2)PF2Ph2][OSO2CF3] 5, [(cAAC)PF2Ph2][OSO2CF3] 6 and [(TripCH2N2(NMe)C2Ph)PF2Ph2][Cl] 7; 4 was not oxidized. These observations are understood by a computational assessment of the average local ionisation potentials at valence shell charge concentrations identified via topological analysis of the electron density.


 Please wait while we load your content...
Please wait while we load your content...