Mechanistic exploration of the copper(i) phosphide synthesis in phosphonium-based and phosphorus-free ionic liquids†
Abstract
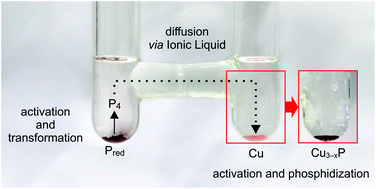
The mechanism of the synthesis of copper(I) phosphide (Cu3−xP) in the ionic liquid (IL) trihexyl(tetradecyl)phosphonium chloride ([P66614][Cl]) was investigated. The phosphide formation is promoted by a transformation of red phosphorus (Pred) into mobile P4 molecules and a surface activation of copper caused by the IL including the Brønsted acidic impurity. The surface activation is important to obtain a quantitative product yield. Moreover, we demonstrate that single-phase Cu3−xP can also be synthesized in the nitrogen-based IL tetrabutylammonium chloride ([N4444][Cl]). Further substitution of the anion of the IL using tetrabutylammonium bromide ([N4444][Br]) or the complete replacement of the IL by a deep eutectic solvent consisting of adipic acid and betaine do not lead to single-phase Cu3−xP. Therefore, the nature of the anions present in the IL also seems to be relevant for the convenient phosphidization reaction.


 Please wait while we load your content...
Please wait while we load your content...