Catalytic fixation of atmospheric carbon dioxide by copper(ii) complexes of bidentate ligands†
Abstract
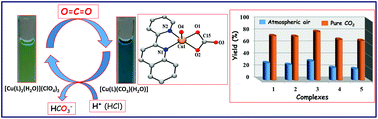
New copper(II) complexes, [Cu(L1)2(H2O)](ClO4)2, 1 [L1 = 2-pyridin-2-yl-quinoline], [Cu(L2)2(H2O)](ClO4)2, 2 [L2 = 2-pyridin-2-yl-quinoxaline], [Cu(L3)2(H2O)](ClO4)2, 3 [L3 = 6,7-dimethyl-2-pyridin-2-yl-quinoxaline], [Cu(L4)2(H2O)](ClO4)2, 4 [L4 = 4-phenyl-2-pyridin-2-yl-quinoline] and [Cu(L5)2(H2O)](ClO4)2, 5 [L5 = 4-phenyl-2-pyridin-2-yl-quinazoline], were synthesized and characterized as catalysts for selective fixation of atmospheric CO2. The molecular structure of 2 was determined by single-crystal X-ray studies and shown to have an unusual trigonal bipyramid geometry (τ, 0.936) around the copper(II) center, with the coordination of two ligand units and a water molecule. The Cu–Nquin (2.040, 2.048 Å) bonds are slightly longer than the Cu–Npyr (1.987 Å) bonds but shorter than the Cu–Owater bond (2.117 Å). Well-defined Cu(II)/Cu(I) redox potentials of around 0.352 to 0.401 V were observed for 1–5 in acetonitrile. The electronic absorption spectra of 1–5 showed ligand-based transitions at around 208–286 nm with a visible shoulder at around 342–370 nm. The d–d transitions appeared at around 750–800 and 930–955 nm in acetonitrile. The rhombic EPR spectra of 1–5 exhibited three different g values gx, 2.27–2.34; gy, 2.06–2.09; and gz, 1.95–1.98 at 70 K. Atmospheric CO2 was successfully fixed by 1–5 using Et3N as a sacrificial reducing agent, resulting in CO32−-bound complexes of type [Cu(L)CO3(H2O)] that display an absorption band at around 614–673 nm and a νst at 1647 cm−1. This CO32−-bound complex of 1 was crystallized from the reaction mixture and it displayed a distorted square pyramidal geometry (τ, 0.369) around the copper(II) center via the coordination of only one ligand unit, a carbonate group, and water molecules. Furthermore, treatment of the carbonate-bound Cu(II) complexes with one equivalent of H+ under N2 atmosphere resulted in the liberation of bicarbonate (HCO3−) and regenerated the parent complexes. These regenerated catalysts were active enough to fix CO2 in eight repeating cycles without any change in efficiency. The fixation of CO2 possibly occurs via the formation of Cu(I)-species, which is accompanied by the formation of an MLCT band at around 450–500 nm. The rates of Cu(I)-species formation, kobs, were determined and found to be 5.41–10.31 × 10−3 s−1 in the presence of Et3N in acetonitrile at 25 °C. Interestingly, the copper(I)-species of 3 has been successfully crystallized and displayed a distorted tetrahedral geometry through the coordination of two units of ligand L3.


 Please wait while we load your content...
Please wait while we load your content...