A series of anionic host coordination polymers based on azoxybenzene carboxylate: structures, luminescence and magnetic properties†
Abstract
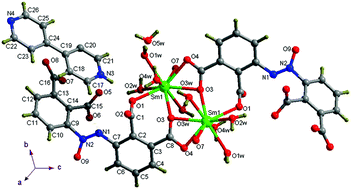
A series of coordination polymers {[Ln(aobtc)(H2O)4]·Hbipy·H2O}n (H4aobtc = azoxybenzene-2,2′,3,3′-tetracarboxylic acid, bipy = 4,4′-bipyridine, and Ln = Sm(1), Eu(2), Gd(3), Tb(4), Dy(5), Er(6)) have been synthesized and characterized systematically. The cationic Hbipy+ guest incorporated polymers are isostructural sets, featuring a one-dimensional (1D) zigzag double chain edifice composed of binuclear clusters [Ln2(H4aobtc)2], with the Hbipy+ guest being located on two sides. These 1D chains are further interlinked into a 2D layer structure, and further extended into a 3D framework through hydrogen bonding interactions. The luminescence emission spectra of polymers 2 and 3 are based on the H4aobtc acid ligands, while 1 and 4 display the characteristic f–f transitions of Ln(III) ions. Magnetic measurements revealed the presence of ferromagnetic behavior in polymer 3. The magnetic behaviors of 4 and 6 are ascribed to the depopulation of the Stark levels and/or weak antiferromagnetic interactions within MOFs at lower temperature. Slow relaxation is observed through the alternating-current susceptibility measurements for 5 at lower temperature, and the coexistence of weak ferromagnetism corresponding to the spin-canting-like behavior.


 Please wait while we load your content...
Please wait while we load your content...