Two-step crystallization of a phase-pure Ln2(OH)5NO3·nH2O layered compound for the smallest Ln ions of Tm, Yb and Lu, anion exchange, and exfoliation
Abstract
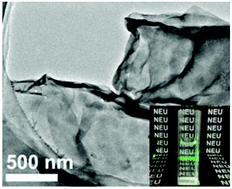
Crystallization of the Ln2(OH)5NO3·nH2O layered hydroxide (LLnH) is the most difficult for the three smallest lanthanide ions of Tm, Yb and Lu. By applying a novel two-step crystallization technique, which involves chemical precipitation at a freezing-temperature of ∼4 °C and subsequent Ostwald ripening at 50 °C for Tm and Yb and 65 °C for Lu, the three compounds have been obtained in a phase-pure form without the use of any mineralizer. The resulting LTmH and LYbH (n ∼ 1.5) were shown to accommodate free NO3− anions in the interlayer gallery, which are readily exchangeable by DS− (C12H25OSO3−). Delamination of the DS− derivatives in formamide produced micron-sized nanosheets of ∼1.7 nm thick. A similar anion exchange was found to hardly proceed for LLuH even at 65 °C, owing to the direct coordination of the interlayer NO3− with the Lu3+ center.


 Please wait while we load your content...
Please wait while we load your content...