Dispersion of Ni2+ ions via acetate precursor in the preparation of NaNiPO4 nanoparticles: effect of acetate vs. nitrate on the capacitive energy storage properties†
Abstract
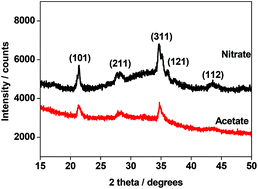
The influence of the precursors on the dispersion of Ni2+ ions and the presence of several other functional groups was investigated in the preparation of sodium nickel phosphate (NaNiPO4) cathode for a supercapacitor study. The dispersion of nickel phases, in the form of nanosheets, is influenced by the type of precursors used in the synthesis. XPS based spectroscopic information on the surface functional groups on NaNiPO4 show differences between the precursors (i.e.) acetate- and nitrate-derived materials. The benefits of using acetate as an alternative to nitrate are explored by using the NaNiPO4 nanoparticles as a cathode for supercapacitor applications. The acetate-derived material exhibits improved electrochemical properties possessing both redox behaviour and double-layer capacitance. The results indicate that the metal acetates are homogenously distributed. Acetate functionalization resulted in an improved capacitance of 90 F g−1 compared with that obtained from the nitrate precursor derived material (58 F g−1). Capacitance retention and high rate capability were also a feature of the acetate-derived material. The sodium nickel phosphate cathode material has provided useful insights on the precursor chemistry in storing renewable energy have been reported for the first time.


 Please wait while we load your content...
Please wait while we load your content...