Stimuli-responsive emissive behavior of 1- and 1,3-connectivities in azulene-based imine ligands: cycloplatination and Pt–Tl dative bond formation†
Abstract
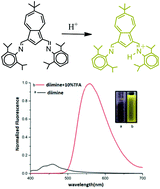
The preparation of two new azulene-based imine ligands N-(2,6-diisopropylphenyl)-6-tBu-1-azulenylmethaneimine, 3, and N-(2,6-diisopropylphenyl)-6-tBu-3-(2,6-diisopropylphenyliminomethyl)-1-azulenylmethaneimine, 4, is described. These imine ligands display stimuli responsive emissive behavior and their fluorescence can be switched on and off by protonation and neutralization with trifluoroacetic acid and trimethylamine, respectively. The cyclometalation of the monoimine ligand by platinum gave the cyclometalated complex [PtMe(SMe2)(3′)], 5, (where the prime denotes the cyclometalated ligand 3). The reaction of 5 with TlPF6 yields the trinuclear bent Pt2Tl complex {[PtMe(SMe2)(3′)]2(μ-Tl)}PF6, 6, via Pt–Tl dative bonds. The compounds 3–6 were characterized using NMR spectroscopy and the solid-state structures of 5 and 6 were further determined by X-ray crystallography. The electronic absorption spectra of the species 3-H+, 4-H+, 5 and 6 were obtained and compared with those observed for the parent species 3 and 4. DFT and TD-DFT calculations are used to elucidate the origin of the electronic transitions in monoimine ligand 3 and its protonated form 3-H+.


 Please wait while we load your content...
Please wait while we load your content...