Hoveyda–Grubbs catalyst analogues bearing the derivatives of N-phenylpyrrol in the carbene ligand – structure, stability, activity and unique ruthenium–phenyl interactions†
Abstract
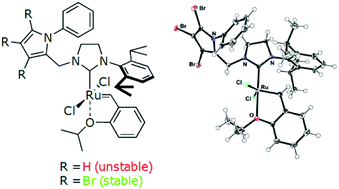
We have synthesized a series of N-phenylpyrrole and N-phenylindole carbenes and used them as ruthenium-ligating moieties in the synthesis of Hoveyda–Grubbs catalyst derivatives. We show that most of these complexes are difficult to synthesize and unstable apart from the N-phenylpyrrole-2,6-diisopropylphenyl ruthenium complex and its perbrominated derivative. These two systems are almost completely inactive in ring-closing metathesis at room temperature and become active only at 80 °C. DFT, SAPT0 and DLPNO-CCSD(T) calculations suggest that the rarely occurring phenyl–ruthenium interactions are responsible for the very slow initiation of these precatalysts at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...