A general strategy to add diversity to ruthenium arene complexes with bioactive organic compounds via a coordinated (4-hydroxyphenyl)diphenylphosphine ligand†
Abstract
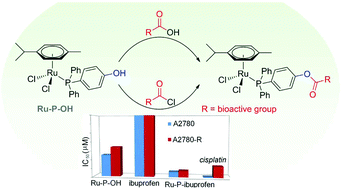
Esterification of (4-hydroxyphenyl)diphenylphosphine, coordinated to the [Ru(η6-p-cymene)Cl2] fragment, allows a series of bioactive carboxylic acids to be introduced directly into the organometallic molecule. Evaluation of the compounds on human ovarian cancer cells reveals synergistic enhancements in their antiproliferative activity relative to their bioactive organic and organometallic precursors.

 Please wait while we load your content...
Please wait while we load your content...