Synthesis, characterization and solution behavior of a systematic series of pentapyridyl-supported RuII complexes: comparison to bimetallic analogs†
Abstract
A series of RuII complexes stabilized with the pentapyridyl ligand Py5Me2 (Py5Me2 = 2,6-bis(1,1-bis(2-pyridyl)ethyl)pyridine) and with an axial X ligand (X = Cl−, H2O, N3−, MeCN) were prepared and characterized in the solid state and in non-aqueous solution. The cyclic voltammograms of these complexes in MeCN reflect a reversible substitution of the axial X ligand with MeCN. Irreversible ligand substitution of [(Py5Me2)RuN3]+ is also observed in propylene carbonate, but only at oxidizing potentials that decompose the azide ligand. The monometallic chloride and azide species are compared with analogous Ru2 metal–metal bonded complexes, which have been reported to undergo irreversible chloride dissociation upon reduction.
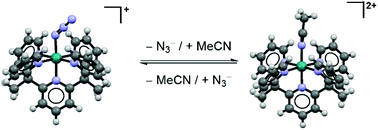


 Please wait while we load your content...
Please wait while we load your content...