A five-coordinate manganese(iii) complex of a salen type ligand with a positive axial anisotropy parameter D†
Abstract
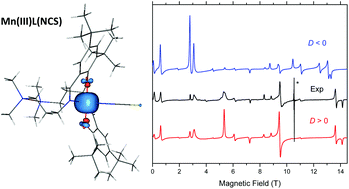
A new high-spin d4 roughly trigonal–bipyramidal (TBP) manganese(III) complex with a salen type ligand (H2L), namely MnL(NCS)·0.4H2O, has been synthesised and characterised by elemental analysis, ESI mass spectrometry, IR and UV-vis spectroscopy, and spectroelectrochemistry. X-ray diffraction analysis revealed an axial compression of the approximate TBP. Temperature dependent magnetic susceptibility and variable-temperature variable-field (VTVH) magnetisation measurements, as well as high-frequency and -field EPR (HFEPR) spectroscopy, were used to accurately describe the magnetic properties of this complex and, in particular, determine the spin Hamiltonian parameters: g-values and the zero-field splitting (ZFS) parameters D and E. The HFEPR spectra allowed the extraction of fourth order ZFS parameters. Quantum chemical calculations reproduced well the electronic and geometric structures of this unusual complex and, in particular, its electronic absorption spectrum along with the spin Hamiltonian parameters.


 Please wait while we load your content...
Please wait while we load your content...