Bis(amidinato)- and bis(guanidinato)silylenes and silylenes with one sterically demanding amidinato or guanidinato ligand: synthesis and reactivity
Abstract
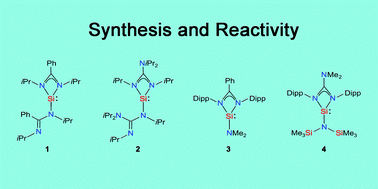
This article deals with the synthesis and reactivity of the bis(amidinato)silylene [iPrNC(Ph)NiPr]2Si (5), the bis(guanidinato)silylene [iPrNC(NiPr2)NiPr]2Si (6), the mono(amidinato)silylene [DippNC(Ph)NDipp]SiNMe2 (Dipp = 2,6-diisopropylphenyl) (8) and the mono(guanidinato)silylene [DippNC(NMe2)NDipp]SiN(SiMe3)2 (9). The reactions studied include (i) nucleophilic substitutions, (ii) Lewis acid/base reactions, (iii) Brønsted acid/base reactions, (iv) oxidative additions (bond activations, small-molecule activations), (v) cycloaddition reactions with unsaturated organic substrates, (vi) reductions and (vii) radical formation by muonium addition. Silylenes 5, 6, 8 and 9 have been demonstrated to display unique reactivity profiles and to be versatile precursors for the synthesis of novel four- or five-coordinate silicon(II) and four-, five- or six-coordinate silicon(IV) complexes with unprecedented structural motifs. This article also deals with the structural characterisation of all the new silicon(II) and silicon(IV) compounds synthesised. Crystal structure analyses and 29Si NMR spectroscopic studies in the solid state and in solution were used to characterise the silicon coordination polyhedra of these compounds.
- This article is part of the themed collection: 2017 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...