Phase-controllable synthesis of cobalt hydroxide for electrocatalytic oxygen evolution†
Abstract
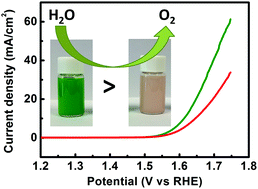
Identification of active sites for oxygen evolution reaction (OER) plays a key role in the design and fabrication of high-performance cobalt-based electrocatalysts. Herein, we report the synthesis of two types of two-dimensional monometallic cobalt hydroxide nanoplates in aqueous solution for OER: α-Co(OH)2 with both Co2+Td and Co2+Oh sites and β-Co(OH)2 with Co2+Oh sites. Electrochemical characterization reveals that α-Co(OH)2 is more active than β-Co(OH)2 towards OER. The better activity can be attributed to the presence of Co2+Td sites in α-Co(OH)2, which are more active than Co2+Oh sites. Our finding clarifies the contribution of the two catalytic sites and helps future rational design of high-performance OER electrocatalysts.
- This article is part of the themed collection: The Role of Inorganic Materials in Renewable Energy Applications


 Please wait while we load your content...
Please wait while we load your content...