Achieving yellow emission by varying the donor/acceptor units in rod-shaped fluorenyl-alkynyl based π-conjugated oligomers and their binuclear gold(i) alkynyl complexes†
Abstract
Fluorenyl-alkynyl based π-conjugated rod-shaped oligomers bearing different central aromatic moieties and functionalizable di-alkynyl termini, such as H–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –H (OH1), H–
–H (OH1), H–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Btz–
–Btz–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –H (OH2) and H–
–H (OH2) and H–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Btd–
–Btd–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –H (OH3) where Fl = 9,9-dioctylfluorene, Btz = N-hexylbenzotriazole, and Btd = benzothiadiazole, were successfully synthesized by a Pd(0) catalyzed Stille coupling protocol. Electron withdrawing benzothiadiazole and benzotriazole as strong to moderate acceptors and fluorene as the donor have been incorporated to adjust the Donor–Acceptor (D–A) strength for fine-tuning the bandgap (Eg) as well as the emission wavelength. The corresponding digold(I) σ-complexes (PPh3)Au–
–H (OH3) where Fl = 9,9-dioctylfluorene, Btz = N-hexylbenzotriazole, and Btd = benzothiadiazole, were successfully synthesized by a Pd(0) catalyzed Stille coupling protocol. Electron withdrawing benzothiadiazole and benzotriazole as strong to moderate acceptors and fluorene as the donor have been incorporated to adjust the Donor–Acceptor (D–A) strength for fine-tuning the bandgap (Eg) as well as the emission wavelength. The corresponding digold(I) σ-complexes (PPh3)Au–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Au(PPh3) (OM1), (PPh3)Au–
–Au(PPh3) (OM1), (PPh3)Au–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Btz–
–Btz–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Au(PPh3) (OM2) and (PPh3)Au–
–Au(PPh3) (OM2) and (PPh3)Au–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Btd–
–Btd–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Fl–
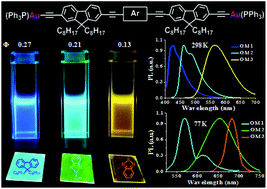
–Fl–![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) –Au(PPh3) (OM3) have also been prepared by a reaction of Au(PPh3)Cl and methanolic NaOMe in DCM with the corresponding alkynyl functionalized oligomers to take advantage of the heavy-atom effect on their emissive properties. The synthesized rod-shaped π-conjugated fluorene based oligomers and their binuclear Au(I) σ-complexes have been unambiguously characterized by various spectroscopic tools such as FTIR and multinuclear NMR as well as MALDI-TOF and CHN analyses. The absorption and emission spectral studies exhibited a progressive red shift with increasing the electron withdrawing character of the central aromatic unit. The rod-like oligomers having alkynyl termini and the corresponding digold(I) complexes are found to be blue, cyan and yellow emissive, demonstrating the fine-tuning of the emission wavelength. Most importantly, the fluorene based π-conjugated yellow light emitters OH3 and OM3 are successfully achieved by varying the donor/acceptor moiety to the fluorenyl-alkynyl backbone. The digold(I) diacetylide organometallic wires exhibit phosphorescence at 77 K in degassed CH2Cl2 due to the efficient intersystem crossing from the S1 to the T1 excited state as induced by heavy atoms.
–Au(PPh3) (OM3) have also been prepared by a reaction of Au(PPh3)Cl and methanolic NaOMe in DCM with the corresponding alkynyl functionalized oligomers to take advantage of the heavy-atom effect on their emissive properties. The synthesized rod-shaped π-conjugated fluorene based oligomers and their binuclear Au(I) σ-complexes have been unambiguously characterized by various spectroscopic tools such as FTIR and multinuclear NMR as well as MALDI-TOF and CHN analyses. The absorption and emission spectral studies exhibited a progressive red shift with increasing the electron withdrawing character of the central aromatic unit. The rod-like oligomers having alkynyl termini and the corresponding digold(I) complexes are found to be blue, cyan and yellow emissive, demonstrating the fine-tuning of the emission wavelength. Most importantly, the fluorene based π-conjugated yellow light emitters OH3 and OM3 are successfully achieved by varying the donor/acceptor moiety to the fluorenyl-alkynyl backbone. The digold(I) diacetylide organometallic wires exhibit phosphorescence at 77 K in degassed CH2Cl2 due to the efficient intersystem crossing from the S1 to the T1 excited state as induced by heavy atoms.


 Please wait while we load your content...
Please wait while we load your content...