Sequential incorporation of metallic cations (Cd2+ and Hg2+) and N-octylamine into titanium phosphate nanoparticles and their subsequent release in acid media†
Abstract
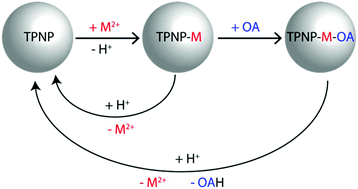
Titanium phosphate nanoparticles, TPNP, consisting of a NaTi2(PO4)3 core and a shell of hydrogen phosphate and dihydrogen phosphate of titanium, undergo fast hydrolysis in water releasing phosphoric acid. This reaction is inhibited in the presence of metallic ions like Cd2+ or Hg2+, which are able to replace the protons of the shell acid phosphates. The amount of the adsorbed metallic cations could be regulated using counterions of different basicity. The resulting nanoparticles also incorporate NH2(CH2)7CH3 (N-octylamine) at room temperature forming N-octylammonium/phosphate ion pairs, but it was found that at higher cation concentration inside the nanoparticle, a lower amount of amine was adsorbed. The metallic cations and N-octylamine are released in acid media, but the starting material is not fully recovered.


 Please wait while we load your content...
Please wait while we load your content...