Synthesis and structural characterisation of the aggregates of benzo-1,2-chalcogenazole 2-oxides†
Abstract
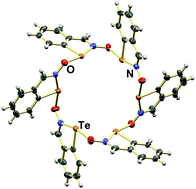
Iodine oxidation of bis[2-(hydroxyiminomethyl)phenyl] dichalcogenides yields benzo-1,2-chalcogenazole 2-oxides. Annulated derivatives of iso-tellurazole N-oxides spontaneously aggregate into cyclic tetra- and hexamers through Te⋯O chalcogen bonding; the structures of the co-crystals with benzene and CH2Cl2 illustrate the ability of these macrocycles to interact with small guest molecules. The selenium congener crystallizes forming a supramolecular polymer. VT NMR indicates that both compounds aggregate in solution but only at low temperature in the selenium case. The different abilities of these molecules to engage in supramolecular interactions are interpreted on the basis of their electronic properties evaluated with DFT-D3 calculations.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...