Magnesium amide catalyzed selective hydroboration of esters†
Abstract
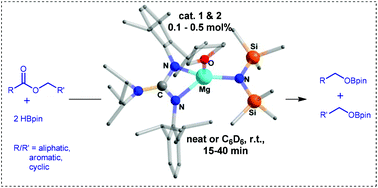
Magnesium amide complexes such as Mg{N(SiMe3)2}2 (1) xylLMgN(SiMe3)2·THF (2) [xylL = ArNC(NiPr2)NAr; (Ar = 2,6-Me2-C6H3)] and dippLMgN(SiMe3)2·THF (3) [dippL = ArNC(NiPr2)NAr; (Ar = 2,6-iPr2-C6H3)] are reported as highly efficient pre-catalysts for the hydroboration of a wide range of esters using pinacolborane (HBpin) under mild reaction conditions. Moreover, we have shown compound 1 catalyzed chemoselective reduction of esters in the presence of other reducible functional groups such as alkene, alkyne and nitro.


 Please wait while we load your content...
Please wait while we load your content...