Synthesis of coenzyme Q0 through divanadium-catalyzed oxidation of 3,4,5-trimethoxytoluene with hydrogen peroxide†
Abstract
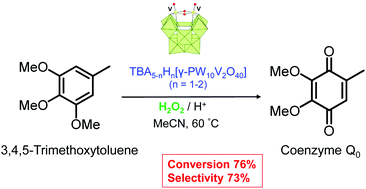
The selective oxidation of methoxy/methyl-substituted arenes to the corresponding benzoquinones has been first realized using aqueous hydrogen peroxide as a green oxidant, acid tetrabutylammonium salts of the γ-Keggin divanadium-substituted phosphotungstate [γ-PW10O38V2(μ-O)2]5− (I) as a catalyst, and MeCN as a solvent. The presence of the dioxovanadium core in the catalyst is crucial for the catalytic performance. The reaction requires an acid co-catalyst or, alternatively, a highly protonated form of I can be prepared and employed. The industrially relevant oxidation of 3,4,5-trimethoxytoluene gives 2,3-dimethoxy-5-methyl-1,4-benzoquinone (ubiquinone 0 or coenzyme Q0, the key intermediate for coenzyme Q10 and other essential biologically active compounds) with 73% selectivity at 76% arene conversion. The catalyst retains its structure under turnover conditions and can be easily recycled and reused without significant loss of activity and selectivity.


 Please wait while we load your content...
Please wait while we load your content...