Anion exchange dynamics in the capture of perchlorate by a cationic Ag-based MOF†
Abstract
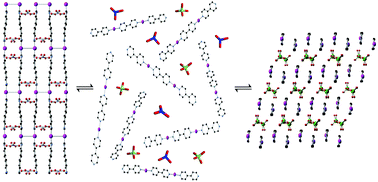
We report a detailed study of the host–guest interaction for a cationic metal–organic framework that can reversibly capture perchlorate. The structural transformation and flexibility of silver 4,4′-bipyridine nitrate (SBN) upon formation of silver 4,4′-bipyridine perchlorate (SBP) was evaluated by monitoring the anion exchange dynamics using a combination of powder X-ray diffraction (PXRD) with multinuclear 13C, 15N and 109Ag solid-state NMR spectra at different time intervals of the anion exchange. The structural transformation from SBN to SBP is complete within 70 minutes and was determined to take place by a solvent-mediated process. This pathway is confirmed by the morphological changes of the two crystalline materials observed by SEM. This key understanding may lead to application of this material towards perchlorate capture.


 Please wait while we load your content...
Please wait while we load your content...